
UV beads experiments

Ultraviolet radiation detection beads are white when not in ultraviolet light, but change colour when exposed to ultraviolet light.
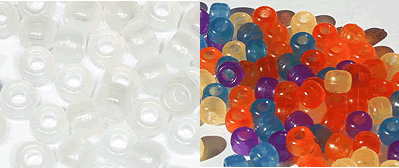
These beads change colour in ultraviolet light
UV beads contain a chemical that is photochromic. This chemical changes colour when exposed to ultraviolet radiation. Normally the beads are colourless, but when ultraviolet light strikes them the molecules become excited and the structure of the molecule changes. This allows the beads to absorb visible light and the colour changes. When taken out of ultraviolet light the molecules go back to the original state and lose the colour. This can happen over and over but it is best to keep the beads out of ultraviolet light as much as possible.
We can use this property in our investigations. Watch the beads changing colour. Now try this for yourself, put some of the beads in sunlight and watch them change colour.
Experiments
Here are some experiments for you to try using UV beads.
Experiment 1: Ultraviolet light